About stem cell culture supernatant
Our cells are damaged every day due to injuries and aging.
What repairs these cells are "stem cells" that differentiate themselves into various cells and promote regeneration.
"Stem cell culture supernatant" is the clear liquid secreted from the upper layer of the precipitate when stem cells are cultured.
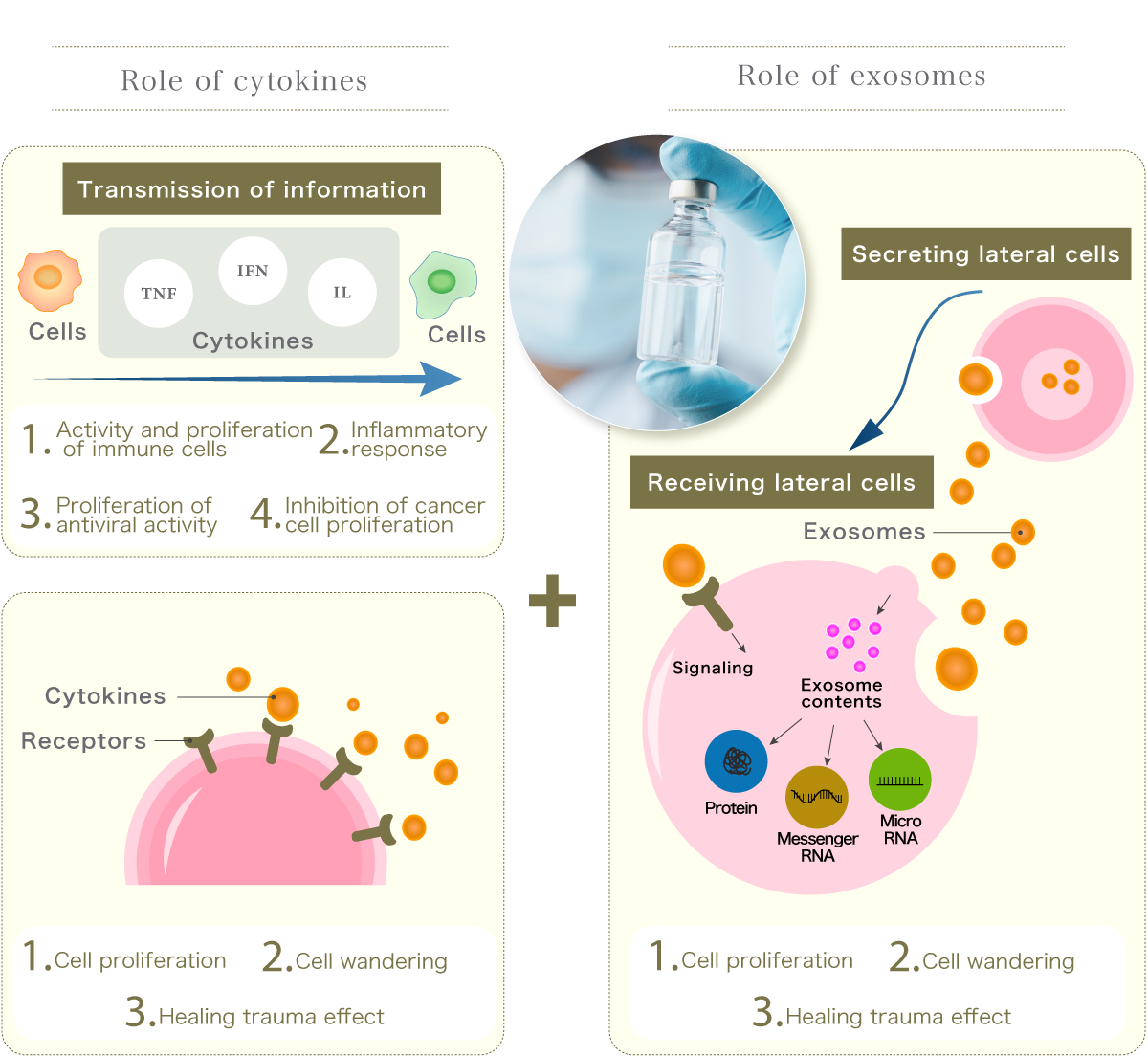
It contains thousands of growth factors (physiologically active substances) such as exosomes and cytokines, which can be expected to produce the same effect as stem cells.
Through the action of exosomes, effects such as cell activation, promotion of tissue regeneration, and improvement of blood circulation can be expected.
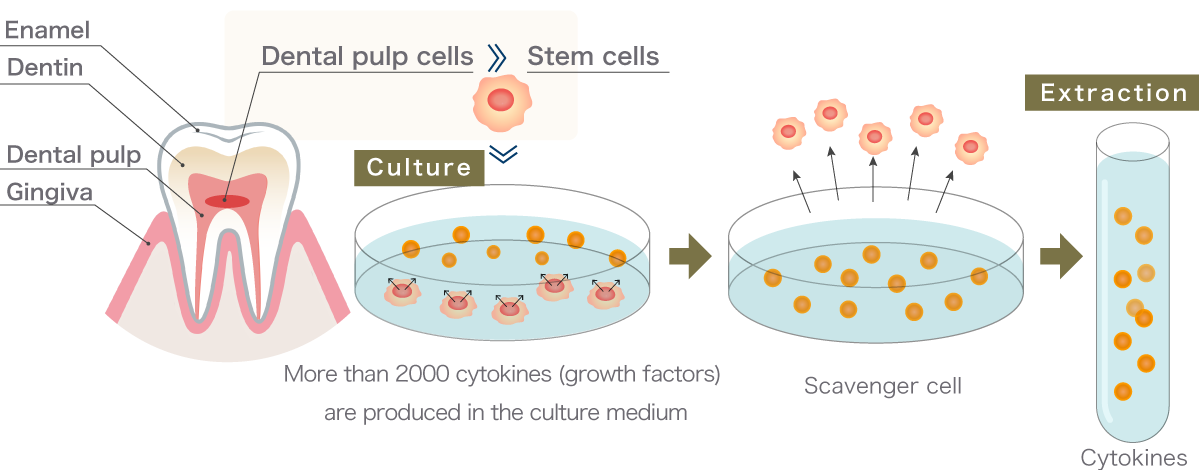
It is also attracting attention in the field of anti-aging. Among the stem cells derived from various human tissues such as fat, bone marrow, and umbilical cord, the pulp stem cells of milk teeth contain the most growth factors.
Reasons to use dental pulp stem cell culture supernatant (exosomes)
Dental pulp is surrounded by hard teeth and is therefore placed in a harsh environment with a very low blood supply compared to other stem cell culture supernatants. It is because of being placed in such an environment that it grows into strong and undamaged cells.
The active capacity of pulp grown in such an environment is very high, with a consequent increase in cell division and proliferation. The greater the proliferation, the greater the variety and concentration of exosomes and cytokines.
U-Factor® in our clinic
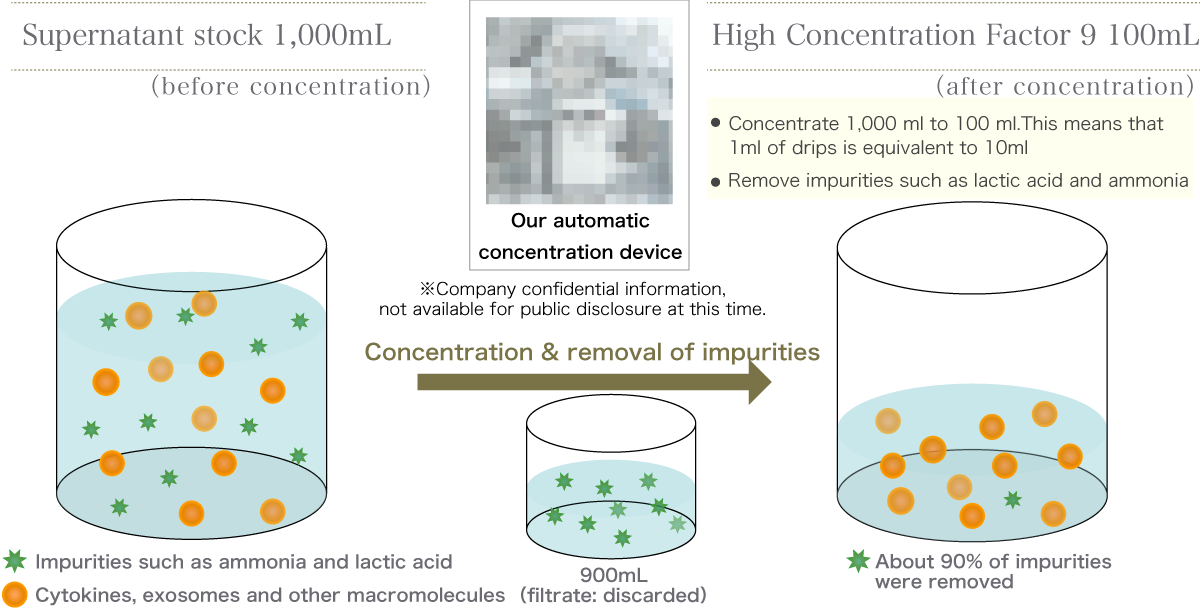
Our "U-Factor®" is a high-quality liquid preparation containing more than 5,000 types of cytokine proteins and a high concentration of exosomes.
4 main points of U-Factor®
and cytokines
High cellular activity in combination with cytokine W
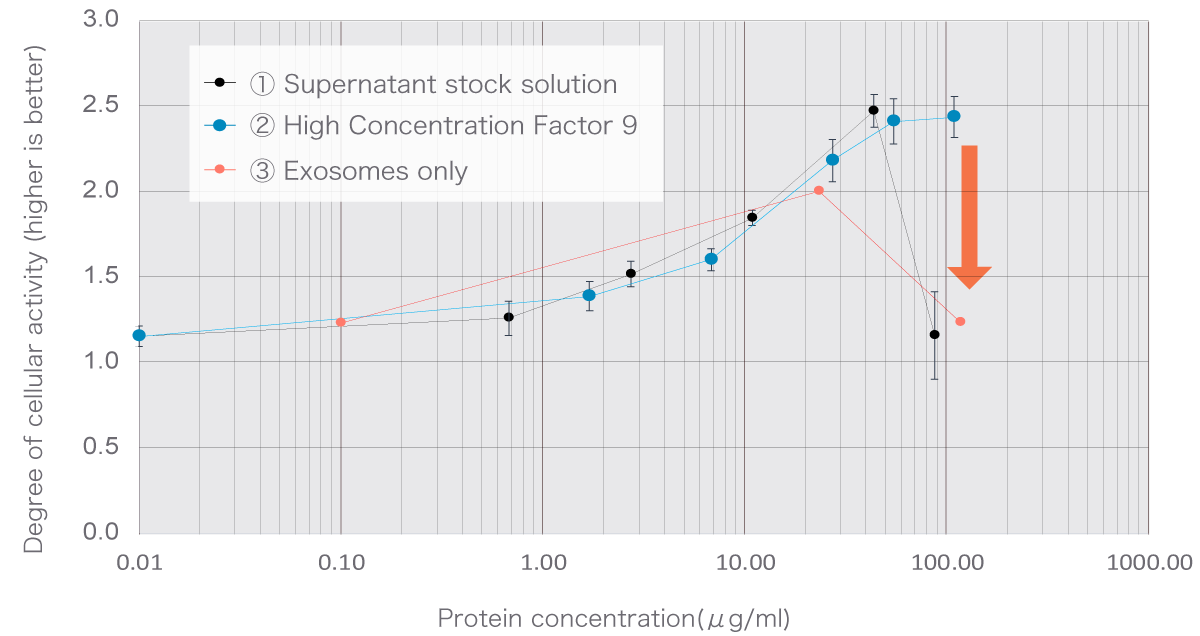
A cell activity test was conducted by varying the concentration of three solutions: stem cell culture supernatant stock solution, our U-Factor®, and exosome-only extract, and found that the U-Factor® showed higher cell activity than the exosome-only extract.
Content of the cell activity test and its results
Experimental content
- Stock solutions for stem cell culture supernatant
- U-Factor®
- Extracts of exosomes only
Experimental results
The stem cell culture supernatant stock solution of 1 and the exosome-only extract of 3 show a drastic decrease in activity at high concentrations, which can be confirmed by the graphs on the right (red arrows).This should be due to the influence of impurities. In addition, it can be confirmed that the degree of activity of the exosome-only extract is also low. Our "U-Factor®" has been proven in experiments to increase cellular activity even at high concentrations.
Reliability
We use pulp stem cells from a defined source.
Our "U-Factor®" collects deciduous teeth from Japanese children and cultures pulp stem cells. It is reviewed by the Ethics Committee, which is indispensable for research in the Faculty of Life Sciences and Medicine that targets human beings, and tooth extraction and pulp tissue collection are performed in an optimal procedure.
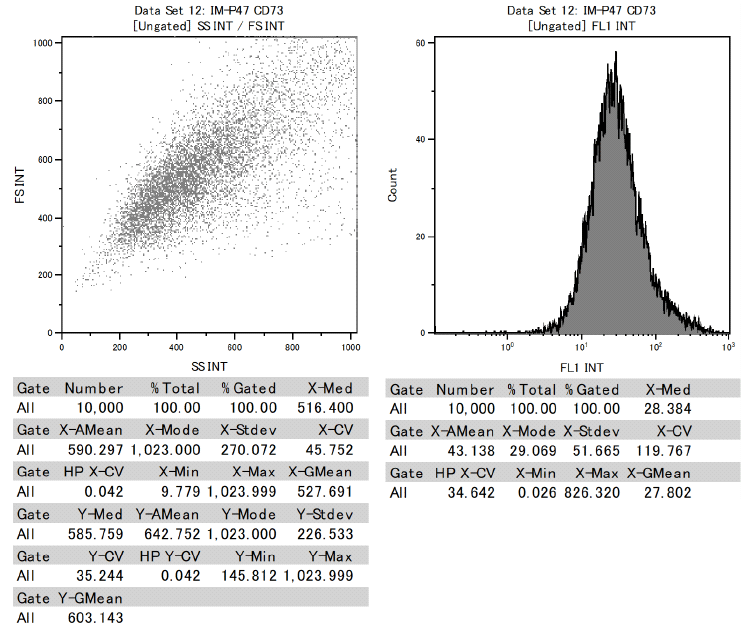
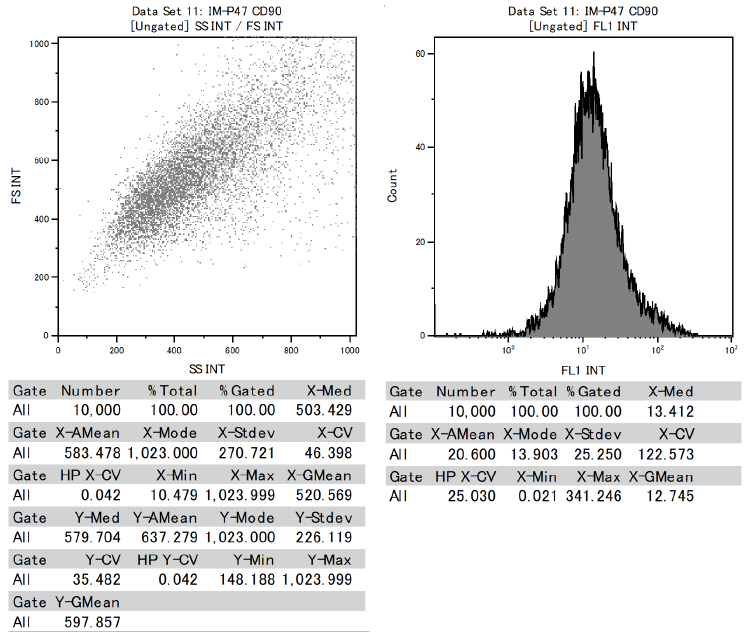
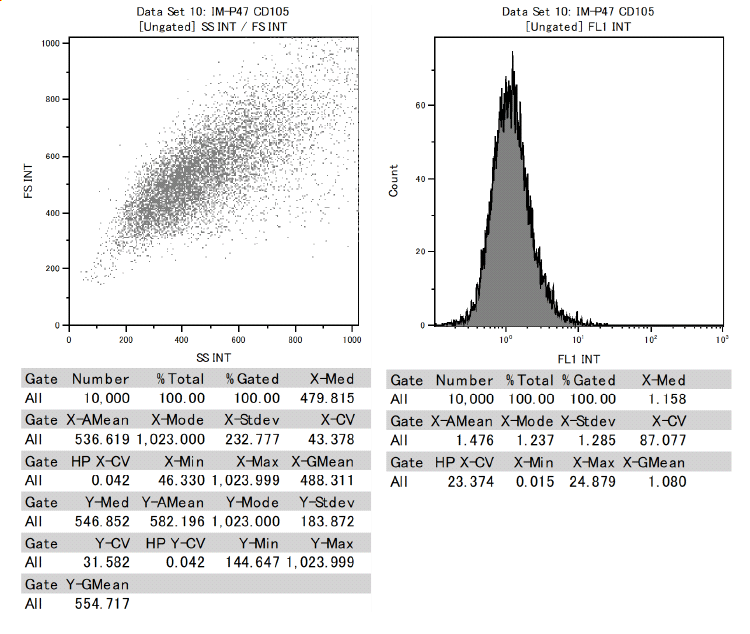
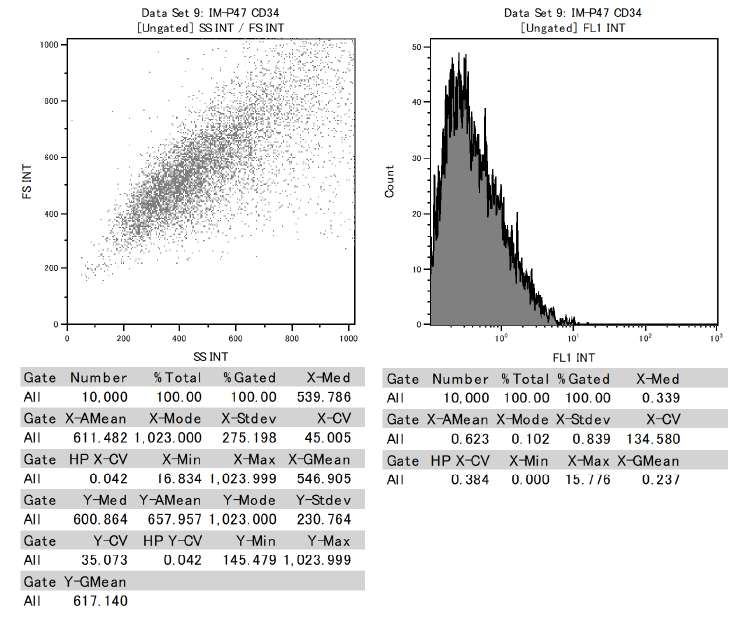
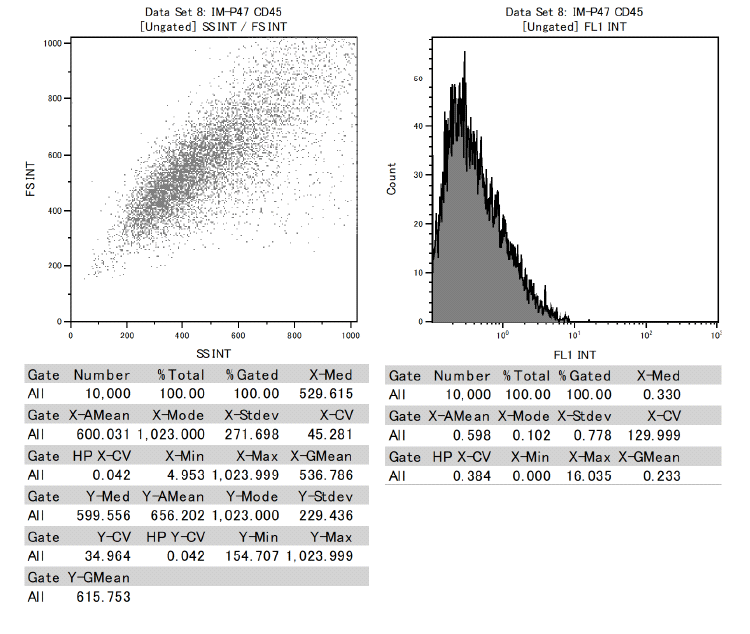
In addition, it was confirmed that dental pulp stem cells were positive for CD73, CD90, and CD105 and negative for CD34 and CD45 among the cell surface markers that indicate MSCs.
This proved to be MSCs with the ability to differentiate into various functional cells.
Results of surface marker test data
It is strictly produced in a GMP-compliant cell processing center.
Compliance with Provincial Orders
- GMPDrug Manufacturing Management and Quality Control Standards
- GCTPProduction management and quality management standards for regenerative medicine and other products
- GQPStandards defining product quality management methods for GQP drugs, quasi-drugs, cosmetics, and regenerative medicine, etc.
- Laws to ensure the safety of regenerative medicine, etc.
Guidelines for Compliance with Specifications and Standards
- ISO(International Organization for Standardization)
An international organization that defines various quality standards. - ICH(International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use) (International Conference on Drug Control Harmonization)
An international organization that promotes guidelines for pharmaceutical standards worldwide - MISEV2018MISEV2018 is a guideline for extracellular vesicle (EV) research standards published by the International Society for Extracellular Vesicles (ISEV) in 2018.

Examination of cells upon receipt and product shipment
Inspection at the time of cell acceptance
- Sterility test
- Endotoxin test/Mycoplasma test
- Virus negative tests (HBs antigen-antibody, HBc antibody, HTLV-I antibody (CLIA), HIV antigen-antibody, syphilis (PRP/TPHA))
Inspection of supernatant upon shipment from the factory
- Sterility test
- Endotoxin test/Mycoplasma test
- Cell-free examination
Total quality management through 4 major safety tests
Extremely pure exosome supernatant liquid “U-Factor®”